This will officially be my last blog post of the year. I thought that this year in Honors Chemistry was very educational and enlightening.
The first day I was in class, I knew it would be different than most classes. One aspect of this class that made it very interesting … and liberating was that cell phones and other electronics were allowed in class–for educational usage only, of course. Normally, these would be prohibited because school administration believes that these are a distraction, promoting cyber-bullying, enable students to cheat on tests, etc. But, cell phones can also be used for good. In this class, cell phones were used to take pictures of lab experiments, projects, take notes, etc. I even used my Kindle Fire to take notes and organize projects, such as the Soap project in the middle of the year.
As a whole, I thought my learning experience was very good. I thought that I did a spectacular job learning in this class. I understood practically everything that I was taught. It just came to me easily. Some of the concepts that I understood easily were conservation of energy, relationships between mass and volume and density, the forming of chemical compounds, predicting the reactants used based on the product formed, and predicting the number of moles produced based on the ratios of the compounds in the balanced equation. I understood that, with density, the greater the mass and the lesser the volume, the greater the density and applied this to identify certain chemical compounds and understood that the chemical composition and the arrangement of particles had to remain the same as well as the density. I could understand the conservation of energy because not only did I know that certain energy could leave or enter the system, but that this decreased or increased the temperature, rearranged the particles, helped to change states, and even that energy could enter or leave the system depending on the chemical reactions. I could tell that based on the reactants in a chemical equation, that they formed this compound, and depending on the charges of the elements, I took advantage of this to find the number of elements required to combine to form a neutrally-charged compound. Based on this, I could calculate the ratios and because of this, I could calculate the ratios of the change in moles in a reaction based on this.
But, one standard I think I struggled with was the difference between was precision and accuracy. I knew that both were important when collecting data and ensuring that I collected measurements accurately, but I couldn't identify the difference. I assumed that they were like interchangeable parts because of this. In our common language, these words mean the same thing. But, in science, it's different. Accuracy is limited based on how we measured it and is associated with human error. But, with precision, it was tough to identify what difference it would make if I had to round a number to the nearest whole number. Whole numbers were expected, but I figured it would be more accurate to say the entire number. But, then accuracy is based on how I measured it, and precision is based on the number of digits. So, I wondered how can something be less precise but just as accurate? It was one of those tough concepts to wrap my head around.
I could do whiteboard problems because the math involved was easy for me to understand. I could calculate the number of moles based on the given grams of that substance and the molar mass of that particular substance so easily and vice versa because I understood they had a proportionate relationship with each other. I often did the whiteboarding, and not did I do a quality job with the whiteboarding, but I also realized that I could solve problems in real life using this technique rather than just memorizing the technique and applying them in just ordinary math problems.
My group and I participated in labs. I thought that I tried my best in the lab experiments. I meticulously ensured that the data I was collecting was correct. I reread all the mathematical procedures I made and made sure I excluded the mass of the beaker when I had to consider the mass of the copper chloride, for example. However, when my energy was wasted on overachieving in APUSH, this was my struggle area. But, I learned a lot from doing the labs. Whenever I dealt with this stress, I was glad we had debriefs afterwards and talk about what we were supposed to learn in the labs. This helped me consider what I should be writing my notes on and how I should write my blog posts and evaluate what I learned.
I also enjoyed the projects in class. I remember the most the thermos project, for example. We were competing to see who could make the most effective thermos, and if one would do very well and had the least temperature change, this would mean they had a great understanding of how energy is conserved and how to keep it this way. I put my best effort in those projects. I often looked for ways to improve and even collected data before I participated in the competition. When I realized the thermos needed more insulation from the inside, I added more and tested it to make sure it gave me results. As soon as I achieved the amount of temperature change I wanted, I stopped and made sure the project was kept intact.
Another aspect of this class that was enlightening was the blog reflections. In my other classes, I would never write on those classes, and if I left my notes in my locker one night before the test, I would be struggling. But, by writing the blog reflections before hand, I can study and review from the blog post to understand the standards that were taught in class. When I write on my blog, I incorporate all the notes I took from class, my experiences with the labs, and what I learned during class. This contributed to my learning because I was less likely to forget what I learned in class even thought math-based classes are my forte and less of a struggle to me than classes like history, which require lots of memorization. The blog writing not only helped me remember the criteria, but it helped me focus on the ares that were the most important. Because there was a word limit (650-750 words), I would always keep my writing within this range, and if it were outside this range, I would omit information that was irrelevant or keep some information since there were other key points I wanted to include. This, though, in spite of the word limit, helped me focus on the concepts I learned in class without getting lost in thought or abstraction since I tend to over-think all the time.
As a matter of fact, over-thinking was one of the things that damaged me this year, in a way, especially when I was stressed out over AP U.S. History. This class was really hard for me because there were so many facts to memorize. This class put me under a lot of pressure because I wanted to get straight A's and have a high GPA, but I always got B's and B-'s. By the time the 2nd semester began, I just resigned and thought to myself, "I give up." This was detrimental because all I could think of was APUSH, and this was bad for me when I had to do lab experiments. With the loss of sleep and focus, I screwed up a lot in labs. I remember when I was trying to do the electrolysis experiment, and I wanted to do it all myself because I was used to doing things the way I wanted to. I struggled, and rather than let anybody help me, I wanted to try to solve the problem myself. As a result, I let my group members down, and I feel bad for doing this. I just hate myself for getting APUSH into my head and making me feel that I couldn't do anything anyone. This negative attitude made me less likely to be able to focus on what I loved the most–chemistry.
If I were to transport myself back in time, I would tell myself to remember the first moment I experience chemistry. As a kid, I played with chemistry sets all the time. I even remember the one time when I watched this one movie where this kid mixes the chemicals incorrectly, and the next thing that happens, it blows up, and his face is covered with black goop. This was funny and made me think that chemistry was so cool and inspiring. And it is. I just wish this year I could hold myself above water and not have to sink so deeply. But now that I have grown, I would tell myself not to overreach and overachieve thinking that I would meet success along the way because my leaps of success in the beginning were short-lived and fleeting. I often dealt with failures, such as the lack of balance and a focus balance between two classes I put all my energy in.
In spite of all this, though, I knew that I wouldn't have to worry about this class much because I given the opportunity to reassess on standards that I wasn't able to study in depth because of other affairs I had to attend to. In the middle of the year, I had to reassess 7 of the 9 questions on this one test because there was a huge APUSH test that I didn't want to fail. So, I didn't get the standards that required me to know the atomic charges of the elements we were studying in class. Nonetheless, the reassessments gave me a chance to study the standards and get better grades. I liked the assessments because I could come into school early at 7 to take them over and not have to worry about missing important information during math or some other class. I was able to find my individual of remembering the charges. I used a mnemonic device for the groups. The first group, for example, was KHANLI for potassium, hydrogen, Li for lithium, and Na reversed for sodium–this helped me remember the +1 charged alkaline metals. I also liked the fact that every time I went to the reassessments, Mr. Abud was always there, and if I needed to, I would ask him questions about certain standards and even tell him what I learned along the way. I thought this was a very helpful and enlightening way to learn chemistry.
Then, within a week after reassessments normally, the reassess grades would replace the ones that I reassess for. For the most part, I would do better and go from a 2 or a 3 to a 4, although there were times when I gone from a 3 to a 2, but those didn't affect my grade much. Nonetheless, I thought the ActiveGrade tool was very good because it didn't base the grades on the number I got correct out of all the possible questions on the test. Each question was graded on a scale of 1 to 4 to determine one's mastery. I thought this tool was helpful in assuring the standards I did and didn't know and helped me take actions accordingly.
I think that this class has exceeded the potential I thought it would be. I can't think of anyway this class can't improve in any way for next year.
I know this blog post is way, way long and exceeds the word limit at 2,164 words. But, in spite of my hypercritical and honest nature, I do have to admit that I enjoyed this class very much and learned much from it. I enjoyed this class because the environment wasn't stressful. I could use a tablet if I wanted to in order to take notes without being yelled at. I liked that I could reassess on standards I felt I struggled with and wanted to improve upon. The class activities, I thought, were engaging and entertaining, but helped me learn in a more … observant fashion. When student volunteers had to align themselves and then move around a certain way to demonstrate the movement of particles in solids, liquids, and gases, I thought it was humorous when the students dumped into each other as if though particles would. This made me chuckle a little and see how particles moved. These are the reasons why I enjoyed this class and will be missing it while I take AP Chemistry next year. As a whole, I would say that my learning experience, on a scale of 1-4, was a solid 4 because I learned every step of the way.
Sunday, June 9, 2013
Sunday, June 2, 2013
May 28-31
This week primarily focused on the Coke bottle lab. In this lab, I had to make the silver precipitate that would form on the inside surface of the bottles.
The first step I took was drinking the pop. This I did, and even though I wanted to rinse it out with water, I was told not to. This makes sense because the water would interfere with the chemical reaction and perhaps cause disassociation.
In every experiment, of course, following the instructions is important. That is why I ensured that I was double-checking the instructions. I made sure that I was using 10 mL of glucose, for example, not 50. The proportion of the reactants is very important in a reaction, and if there is too much of one reactant, then not enough of the excess reactant will react. It could ruin the experiment.
In the experiment, I measured out 10 mL of glucose and put it in the bottle.
I then got the silver nitrate and volumed it at 40 mL, but did not put it in with the glucose since the instructions said not to. I thought that since the silver nitrate would have to go in with the glucose eventually, what would happen if it were mixed with glucose first?
The next step I took in the experiment was I added drops of ammonia to the silver nitrate. As soon as I did this, a brown precipitate form, but to dissolve it, I added more ammonia. This I think happened because the silver nitrate was an aqueous solution, and the ammonia ions separated the silver nitrate, hence forming the precipitate. The more ammonia ions there were, logically, the precipitate would eventually dissolve depending on the number of ions it needs. This then formed silver ammonium (Ag(NH3)2).+
Then, I added the 15 mL of potassium hydroxide to the silver ammonium complex. Then, I combined this with the glucose inside the bottle. In order for the reaction to occur, though, I had to shake the bottle in order for the particles to collide together, hence the electrons could be transferred from one element to another. Hence, motion also plays a role in catalyzing chemical reactions.
 The bottle, of course, needed to have a stopper so that none of the substance would come out. So, I sealed it carefully before I shook the bottle. As I continued shaking the bottle, I noticed that it was turning black. As the instructions said, fine particles of any metal are black. This was how it appeared when I performed the experiment, so I knew I was doing the experiment correctly. As soon as the coating appeared to be even, I stopped shaking, and now I'm waiting for the solution to solidify to form the silver layer inside.
The bottle, of course, needed to have a stopper so that none of the substance would come out. So, I sealed it carefully before I shook the bottle. As I continued shaking the bottle, I noticed that it was turning black. As the instructions said, fine particles of any metal are black. This was how it appeared when I performed the experiment, so I knew I was doing the experiment correctly. As soon as the coating appeared to be even, I stopped shaking, and now I'm waiting for the solution to solidify to form the silver layer inside.
This reaction, overall, is known as the Tollen's test, which is used to identify the aldehydes in the reaction. The mirror forms when metallic silver deposits on the inner surface of a glass container solidifies, hence forming the silver precipitate. In this experiment, the aldehyde was glucose. When it combined with the ammonia complex of silver in an aqueous solution, the silver was eventually disassociated from the rest of the complex, hence isolating the ions to form the silver precipitate.
This would explain how mirrors are made. In normal mirrors, the silver is used, and since the test forms reflective silver, light can reflect off of it due to its physical and chemical properties, which explains why mirrors often look silver, or depending on the color of the metal used like copper, the light reflecting off the mirror makes it appear to be that particular color.
This week, I also reviewed over the whole course of the semester. The concepts that I learned this semester played into this lab. One of the basic concepts that played a role in this lab were the properties of matter. In this lab, I observed that the silver nitrate, the potassium hydroxide, and the ammonia were all liquids. This was different than most of the chemical reactions because if look back on the copper chloride experiment, for example, a solid (copper) reacted with a liquid (chloride) in an aqueous solution in water. Water happened to separated the ions through disassociation. But, in this one, all were liquid, which may have played a role because they didn't require a solvent to form an aqueous solution. Rather, it required the glucose to react to help form the silver precipitate.
The first step I took was drinking the pop. This I did, and even though I wanted to rinse it out with water, I was told not to. This makes sense because the water would interfere with the chemical reaction and perhaps cause disassociation.
In every experiment, of course, following the instructions is important. That is why I ensured that I was double-checking the instructions. I made sure that I was using 10 mL of glucose, for example, not 50. The proportion of the reactants is very important in a reaction, and if there is too much of one reactant, then not enough of the excess reactant will react. It could ruin the experiment.
In the experiment, I measured out 10 mL of glucose and put it in the bottle.
I then got the silver nitrate and volumed it at 40 mL, but did not put it in with the glucose since the instructions said not to. I thought that since the silver nitrate would have to go in with the glucose eventually, what would happen if it were mixed with glucose first?
The next step I took in the experiment was I added drops of ammonia to the silver nitrate. As soon as I did this, a brown precipitate form, but to dissolve it, I added more ammonia. This I think happened because the silver nitrate was an aqueous solution, and the ammonia ions separated the silver nitrate, hence forming the precipitate. The more ammonia ions there were, logically, the precipitate would eventually dissolve depending on the number of ions it needs. This then formed silver ammonium (Ag(NH3)2).+
Then, I added the 15 mL of potassium hydroxide to the silver ammonium complex. Then, I combined this with the glucose inside the bottle. In order for the reaction to occur, though, I had to shake the bottle in order for the particles to collide together, hence the electrons could be transferred from one element to another. Hence, motion also plays a role in catalyzing chemical reactions.
 The bottle, of course, needed to have a stopper so that none of the substance would come out. So, I sealed it carefully before I shook the bottle. As I continued shaking the bottle, I noticed that it was turning black. As the instructions said, fine particles of any metal are black. This was how it appeared when I performed the experiment, so I knew I was doing the experiment correctly. As soon as the coating appeared to be even, I stopped shaking, and now I'm waiting for the solution to solidify to form the silver layer inside.
The bottle, of course, needed to have a stopper so that none of the substance would come out. So, I sealed it carefully before I shook the bottle. As I continued shaking the bottle, I noticed that it was turning black. As the instructions said, fine particles of any metal are black. This was how it appeared when I performed the experiment, so I knew I was doing the experiment correctly. As soon as the coating appeared to be even, I stopped shaking, and now I'm waiting for the solution to solidify to form the silver layer inside.This reaction, overall, is known as the Tollen's test, which is used to identify the aldehydes in the reaction. The mirror forms when metallic silver deposits on the inner surface of a glass container solidifies, hence forming the silver precipitate. In this experiment, the aldehyde was glucose. When it combined with the ammonia complex of silver in an aqueous solution, the silver was eventually disassociated from the rest of the complex, hence isolating the ions to form the silver precipitate.
This would explain how mirrors are made. In normal mirrors, the silver is used, and since the test forms reflective silver, light can reflect off of it due to its physical and chemical properties, which explains why mirrors often look silver, or depending on the color of the metal used like copper, the light reflecting off the mirror makes it appear to be that particular color.
This week, I also reviewed over the whole course of the semester. The concepts that I learned this semester played into this lab. One of the basic concepts that played a role in this lab were the properties of matter. In this lab, I observed that the silver nitrate, the potassium hydroxide, and the ammonia were all liquids. This was different than most of the chemical reactions because if look back on the copper chloride experiment, for example, a solid (copper) reacted with a liquid (chloride) in an aqueous solution in water. Water happened to separated the ions through disassociation. But, in this one, all were liquid, which may have played a role because they didn't require a solvent to form an aqueous solution. Rather, it required the glucose to react to help form the silver precipitate.
Sunday, May 26, 2013
May 20-24
This week, I learned about endothermic and exothermic reactions. Not only did I learn this, but I also revisited some content from Unit 6 pertaining to the energy bar graphs and even came up with the concept of the transfer of chemical energy through these reactions.
But, first, let's first revisit the iron and copper (II) chloride lab. One of the observations I made during the lab was that as the iron nail reacted with the CuCl2, the temperature increased. I knew the temperature was increasing because when I felt the beaker, it felt warmer than previously. Therefore, a chemical reaction occurred in the beaker. Not only this, but energy was also transferred from the aqueous solution to the surface of the glass beaker.
As energy transferred out of the system, the atoms were rearranged. This makes sense because energy was required to break apart the copper and the chlorine. Hence, copper and chlorine were dissociated and formed into neutrally charged ions.
Initially, copper had a charge of +2 while Cl2 had a charge of -1 per chlorine atom. When they were separated, though, the energy transferred to them, dissociating them and transferring electrons. Since the copper has a positive charge (as all metals do), it needed to gain 2 e- (electrons are negatively charged) to neutralize its charge. Hence, the diatomic chlorine lost 2 e-, making it more positive and neutrally charged.
In order to bond the two together, though, they are both put into water in an electrolysis kit to form an aqueous solution. As I've mentioned before, the copper and the chloride separated from each other due to energy. But, where did the energy come from? Obviously, whatever was hooked up to the electrolysis kit! In this case, it was the genecon. The electrons from chloride to copper were transferred from the chloride (right) to the copper (left). In one of the previous, I questioned what would happen if the genecon were turned counter clockwise? It turns out that it doesn't really matter because the electrons are still transferred from the chloride to the copper–just in a counter clockwise direction. Either way, the genecon enabled the electrical current it produced to carry the electrons from one substance to another to neutralize the charges and to separate.
As a result, we came to the conclusion that chemical energy inherently was a major part in this experiment. From this, we learned how to use the LOL diagram charts to graph it. But, the question is how and what would the changes in the ph and th accounts be?
We know that from the experiment with the iron nail and the copper chloride that the iron chloride formed and that the copper precipitate formed. When they reacted, energy escaped the system. But how? When they chemically reacted, a chemical reaction occurred, but of course was used up as soon as all the moles of iron and copper chloride that could possibly react together (of course, the amount is limited depending on the ratios). Hence, after the reaction occurred, chemical energy escaped from the system. The same way worked with thermal energy. As the reaction occurred, the beaker got warmer. Hence, the chemical energy that escaped the system was converted into thermal energy, which of course also was released from the system since the beaker gradually cooled down afterwards.
As I said up top, I would discuss endothermic and exothermic reactions. What occurred here was clearly an exothermic reaction because energy was released from this system. In the reaction, it took more energy to bond together the copper chloride than the iron chloride. Of course, one could say that it took more energy to bond iron and chloride or else they wouldn't be bonded. These both sound plausible, but what is actually happening? Let's considered that in the experiment, there was an excess amount of iron left, which meant that enough energy was used to separate the copper and the chloride and bond the iron to the chloride. Of course, it took more energy to separate the copper and the chloride because it requires more energy to bond than iron chloride. This makes sense because copper has a huger molar mass (63.54 g/mol) than iron (55.85 g/mol). Logically, it would take more energy to take apart something with a greater mass just as much as it would take a greater force (twice as much) to move an object of 200 lbs from x distance and half as much required for the larger object to push the 100 lb object. I noticed a trend that the greater the molar mass, the greater the number of electrons. This would explain why more energy was used to bond copper chloride than iron chloride.
This would also make sense because once the electricity passes through iron, it becomes magnetic, and since less energy is required to bond iron to chloride, this would mean that some of the energy is being used to make it magnetic. However, since some chemical energy has been used up to form a new substance and rearrange the particles, it becomes less magnetic than iron itself.
Therefore, the reactants in an exothermic reaction have greater chemical energy than the energy in the products because some of that energy has already been used up.
This week, however, we have not really looked into endothermic reactions, but endothermic reactions are the opposite. Instead of leaving the system, energy enters the system. This would mean that there was less chemical and thermal energy to begin with, but when the reaction occurred, energy was needed in the system, so the products made required more energy than the reactants. When the alka seltzer is put in the water, for example, a gas is created. But, was it created from the energy that entered the system? This is where it gets confusing. Hopefully, this is something I can put energy into studying next week and for AP Chem next year.
But, first, let's first revisit the iron and copper (II) chloride lab. One of the observations I made during the lab was that as the iron nail reacted with the CuCl2, the temperature increased. I knew the temperature was increasing because when I felt the beaker, it felt warmer than previously. Therefore, a chemical reaction occurred in the beaker. Not only this, but energy was also transferred from the aqueous solution to the surface of the glass beaker.
As energy transferred out of the system, the atoms were rearranged. This makes sense because energy was required to break apart the copper and the chlorine. Hence, copper and chlorine were dissociated and formed into neutrally charged ions.
Initially, copper had a charge of +2 while Cl2 had a charge of -1 per chlorine atom. When they were separated, though, the energy transferred to them, dissociating them and transferring electrons. Since the copper has a positive charge (as all metals do), it needed to gain 2 e- (electrons are negatively charged) to neutralize its charge. Hence, the diatomic chlorine lost 2 e-, making it more positive and neutrally charged.
 |
| On top, copper is collected on left tube. On bottom, copper is collected on right tube. |
In order to bond the two together, though, they are both put into water in an electrolysis kit to form an aqueous solution. As I've mentioned before, the copper and the chloride separated from each other due to energy. But, where did the energy come from? Obviously, whatever was hooked up to the electrolysis kit! In this case, it was the genecon. The electrons from chloride to copper were transferred from the chloride (right) to the copper (left). In one of the previous, I questioned what would happen if the genecon were turned counter clockwise? It turns out that it doesn't really matter because the electrons are still transferred from the chloride to the copper–just in a counter clockwise direction. Either way, the genecon enabled the electrical current it produced to carry the electrons from one substance to another to neutralize the charges and to separate.
As a result, we came to the conclusion that chemical energy inherently was a major part in this experiment. From this, we learned how to use the LOL diagram charts to graph it. But, the question is how and what would the changes in the ph and th accounts be?
We know that from the experiment with the iron nail and the copper chloride that the iron chloride formed and that the copper precipitate formed. When they reacted, energy escaped the system. But how? When they chemically reacted, a chemical reaction occurred, but of course was used up as soon as all the moles of iron and copper chloride that could possibly react together (of course, the amount is limited depending on the ratios). Hence, after the reaction occurred, chemical energy escaped from the system. The same way worked with thermal energy. As the reaction occurred, the beaker got warmer. Hence, the chemical energy that escaped the system was converted into thermal energy, which of course also was released from the system since the beaker gradually cooled down afterwards.
As I said up top, I would discuss endothermic and exothermic reactions. What occurred here was clearly an exothermic reaction because energy was released from this system. In the reaction, it took more energy to bond together the copper chloride than the iron chloride. Of course, one could say that it took more energy to bond iron and chloride or else they wouldn't be bonded. These both sound plausible, but what is actually happening? Let's considered that in the experiment, there was an excess amount of iron left, which meant that enough energy was used to separate the copper and the chloride and bond the iron to the chloride. Of course, it took more energy to separate the copper and the chloride because it requires more energy to bond than iron chloride. This makes sense because copper has a huger molar mass (63.54 g/mol) than iron (55.85 g/mol). Logically, it would take more energy to take apart something with a greater mass just as much as it would take a greater force (twice as much) to move an object of 200 lbs from x distance and half as much required for the larger object to push the 100 lb object. I noticed a trend that the greater the molar mass, the greater the number of electrons. This would explain why more energy was used to bond copper chloride than iron chloride.
This would also make sense because once the electricity passes through iron, it becomes magnetic, and since less energy is required to bond iron to chloride, this would mean that some of the energy is being used to make it magnetic. However, since some chemical energy has been used up to form a new substance and rearrange the particles, it becomes less magnetic than iron itself.
Therefore, the reactants in an exothermic reaction have greater chemical energy than the energy in the products because some of that energy has already been used up.
This week, however, we have not really looked into endothermic reactions, but endothermic reactions are the opposite. Instead of leaving the system, energy enters the system. This would mean that there was less chemical and thermal energy to begin with, but when the reaction occurred, energy was needed in the system, so the products made required more energy than the reactants. When the alka seltzer is put in the water, for example, a gas is created. But, was it created from the energy that entered the system? This is where it gets confusing. Hopefully, this is something I can put energy into studying next week and for AP Chem next year.
Sunday, May 19, 2013
May 13-17
I learned more on not just how to use stoichiometry theoretically but practically as well. This week, my group and I worked on chemically combining iron and copper chloride for this lab.
The challenge was to determine how to balance the equation. Before we proceeded, of course, we massed out the nail to ensure accuracy and then we measured out the required volume of copper chloride. These steps were important to take considering the ratios of the reactants in the balanced equation. If the excess ingredient was, say the iron nail, then this means that some of it is left, which means that too much iron was used; hence, iron would be the excess ingredient. This would make copper chloride the limited reactant was enough of it was used to form the precipitate.
But, the question was what was the precipitate that would form? Initially, I guessed that it would form iron oxide because water was also used in the reaction as well. I figured because of the oxygen in water and my previous experiences with riding my bike in the puddles and leaving my brakes to rust, iron oxide would form.
Hence, the original chemical equation we came up with is:
When the reactants were reacted together, the rust on the nail came right off the nail. This supported my new hypothesis until I realized that the ions in water weren't what separated the iron and the copper chloride because when we performed the fire test (assuming that H2 gas was created, it would combust), nothing happened. Therefore, the water did not react with the iron. So, what did then?
But, my theory before that was even more ridiculous. I believed that the H2 and the Cl2 reacted together to form hydrogen chloride, but hydrogen is one of those weird elements. Even though hydrogen and oxygen are both gases, they can combine to form liquid. Since a liquid has stronger attraction between the particles than a gas, this would disprove my preposterous theory because water has a greater electronegativity than hydrogen chloride. The greater the pull on the electrons is, the greater the attraction is also.
I, was, however, considering that the iron oxidized because the red rust came off the nail, hence I still concluded that the oxygen from water didn't combine with it, but just reacted it with it. This, however, is not a plausible theory because when reactants react with each, they always form a new chemical compound or chemical compounds. Secondly, the copper chloride solution did change color, which is a sign it chemically reacted with something. Initially, the copper chloride was cyan, but when it reacted with the iron, the color changed to a greenish blue to green to greenish red.
I then noticed that the rust from the nail is the same color as the red solution. Since water wouldn't change the color of the solution (after all, it is colorless) nor the color of copper chloride (since it already turned cyan when it reacted to water), I concluded that it reacted with the iron. But, without the water, the chemical reaction couldn't react between iron and copper chloride because the copper chloride was powdery and the iron was metal. It doesn't make sense to combine both solids together and expect them to form new chemical compounds. The water helped to disassociate the copper chloride and turn it to a liquid so that it could transfer the electrons as it reacted with a positively charged metal. This is what happened when the copper chloride and the iron reacted together.
With this information, I changed the chemical equation and decided that iron chloride formed, and that the precipitate that formed was the copper. I noticed that to start out with, iron was neutrally charged since it wasn't chemically combined to any element and that the copper precipitate that formed was also neutrally charged. Because of this, a single replacement reaction occurred. The iron and the copper swapped places with diatomic chloride.
The stoichiometry was the last step of this lab. The amount of moles for every amount of grams had to be calculated for the reactants. First, let's consider the amount of mass the iron had before hand. Initially, it started out with 30 grams. For every 30 g, there are 0.54 moles since the atomic mass of iron is 55.85 g for every mole. The copper (II) chloride was massed at 7.5 grams. For every 7.5 g, there are 0.06 moles since the atomic mass of copper chloride is 134.45 g for every mole. Based on the ratios of the products (2 FeCl3: 3 Cu) to the reactants (2 Fe: 3CuCl2), 0.04 moles of copper (III) chloride and 0.06 moles of iron are created.
The challenge was to determine how to balance the equation. Before we proceeded, of course, we massed out the nail to ensure accuracy and then we measured out the required volume of copper chloride. These steps were important to take considering the ratios of the reactants in the balanced equation. If the excess ingredient was, say the iron nail, then this means that some of it is left, which means that too much iron was used; hence, iron would be the excess ingredient. This would make copper chloride the limited reactant was enough of it was used to form the precipitate.
But, the question was what was the precipitate that would form? Initially, I guessed that it would form iron oxide because water was also used in the reaction as well. I figured because of the oxygen in water and my previous experiences with riding my bike in the puddles and leaving my brakes to rust, iron oxide would form.
Hence, the original chemical equation we came up with is:
When the reactants were reacted together, the rust on the nail came right off the nail. This supported my new hypothesis until I realized that the ions in water weren't what separated the iron and the copper chloride because when we performed the fire test (assuming that H2 gas was created, it would combust), nothing happened. Therefore, the water did not react with the iron. So, what did then?
But, my theory before that was even more ridiculous. I believed that the H2 and the Cl2 reacted together to form hydrogen chloride, but hydrogen is one of those weird elements. Even though hydrogen and oxygen are both gases, they can combine to form liquid. Since a liquid has stronger attraction between the particles than a gas, this would disprove my preposterous theory because water has a greater electronegativity than hydrogen chloride. The greater the pull on the electrons is, the greater the attraction is also.
I, was, however, considering that the iron oxidized because the red rust came off the nail, hence I still concluded that the oxygen from water didn't combine with it, but just reacted it with it. This, however, is not a plausible theory because when reactants react with each, they always form a new chemical compound or chemical compounds. Secondly, the copper chloride solution did change color, which is a sign it chemically reacted with something. Initially, the copper chloride was cyan, but when it reacted with the iron, the color changed to a greenish blue to green to greenish red.
 |
| Iron (III) Chloride and Iron (II) Chloride |
I then noticed that the rust from the nail is the same color as the red solution. Since water wouldn't change the color of the solution (after all, it is colorless) nor the color of copper chloride (since it already turned cyan when it reacted to water), I concluded that it reacted with the iron. But, without the water, the chemical reaction couldn't react between iron and copper chloride because the copper chloride was powdery and the iron was metal. It doesn't make sense to combine both solids together and expect them to form new chemical compounds. The water helped to disassociate the copper chloride and turn it to a liquid so that it could transfer the electrons as it reacted with a positively charged metal. This is what happened when the copper chloride and the iron reacted together.
With this information, I changed the chemical equation and decided that iron chloride formed, and that the precipitate that formed was the copper. I noticed that to start out with, iron was neutrally charged since it wasn't chemically combined to any element and that the copper precipitate that formed was also neutrally charged. Because of this, a single replacement reaction occurred. The iron and the copper swapped places with diatomic chloride.
The stoichiometry was the last step of this lab. The amount of moles for every amount of grams had to be calculated for the reactants. First, let's consider the amount of mass the iron had before hand. Initially, it started out with 30 grams. For every 30 g, there are 0.54 moles since the atomic mass of iron is 55.85 g for every mole. The copper (II) chloride was massed at 7.5 grams. For every 7.5 g, there are 0.06 moles since the atomic mass of copper chloride is 134.45 g for every mole. Based on the ratios of the products (2 FeCl3: 3 Cu) to the reactants (2 Fe: 3CuCl2), 0.04 moles of copper (III) chloride and 0.06 moles of iron are created.
Sunday, May 12, 2013
May 6-10
This week was anther continuation of stoichiometry and how to apply it. Last week, I said that stoichiometry can be used to calculate the number of moles of the products will be created based on the ratios and number of moles of the reactants. But, what if there were a limited or excess reactant?
In this case, the same steps can be applied. The only difference is that too much of a product will be left over while not enough or just enough of another product can be used to make the products.
The first step in finding the excess and limited reactants is to know the ratios of the compounds, which can be calculated by balancing the chemical equation.
The second step is to do some mental math. If the ratio of the reactants was 1:3 and the ratio of the actual amount of elements is different from that, then this will affect the yield that will be produced but keep the ratios the same or a different compound will form if the ratios aren't proportionate. For example, if the actual ratio were 1:2, then the larger number of reactants would be used up to maintain the ratio of reactants and to leave the smaller number of reactants left. Since all of 2 would be used up (33% less than 3), 33% of 1 would be used up (leaving 2/3 of the reactant left). The 2/3:2 is equivalent to a 1:3 ratio since 2/3 is 1/3 of 2. By analyzing the changing ratios from the original ratio of compounds to the actual compounds, you can find the excess ingredient as well as the limited reactant.
The above steps are how you solve simple stoichiometry problems. But, complicated ones may involve converting x g of this element to moles. In order to do so, you need to know the molar mass of the element, which can be found on the Periodic Table of Elements, hence making the Periodic Table of Elements quite useful. If you don't see molar mass in the squares, look for atomic mass because the two mean the same thing. The only difference is that atomic mass isn't necessarily attached to moles. It just determines how much mass an element contains. But, the molar mass is associated with the atomic mass per mole (6.02 * 10^23 particles).
Once you have found the molar mass, you can set up a proportion for the molar mass and the given mass of the element. Or, you could divide or multiply the given mass by the molar mass to find the number of moles depending on if the given mass greater than or less than the molar mass. If less than the molar mass, then it must be less than one mole, so you would simply divide the given mass by the molar mass. But, if the given mass is greater than the molar mass, then the molar mass must be divided by the given mass to find the number of moles.
Another complicated process of stoichiometry is if the compound contained more than one element. In this case, add up all the atomic masses together to find the molar mass. Another point to bring up is that molar mass can represent the total atomic mass of the compound whereas the atomic mass represents only one element.
One thing that wasn't mentioned was that stoichiometry only calculates the yield that could be formed. Theoretically speaking, for example, if an experiment involved combining a certain amount of sodium chloride with a certain amount of aluminum sulfate but you have a limited or excess amount of a compound, this will affect the yield you will produce. For example, if you could theoretically produce thrice the amount of sodium sulfate to twice the amount of aluminum chloride using 6NaCl + Al^2(SO4)3 ------- 3Na2(SO4) + 2Al(Cl3) but produced only 200g of sodium sulfate instead of the required 300g, then the yield is about 67%, or a ratio of 2:3. The yield percentage between moles and grams is proportional.
In order to calculate the yield produced, you must go through all the steps of stoichiometry. At the end, once you know the moles of a product produced compared to what could have been produced, you can divide the actual mass or moles of a compound by the theoretical mass or moles. Think of taking a test, and the test consists of 100 questions, but you only get 80% correct. The percentage of questions you got right are 80%. This concept can be applied to find the yield percentage of the products produced.
In this case, the same steps can be applied. The only difference is that too much of a product will be left over while not enough or just enough of another product can be used to make the products.
The first step in finding the excess and limited reactants is to know the ratios of the compounds, which can be calculated by balancing the chemical equation.
The second step is to do some mental math. If the ratio of the reactants was 1:3 and the ratio of the actual amount of elements is different from that, then this will affect the yield that will be produced but keep the ratios the same or a different compound will form if the ratios aren't proportionate. For example, if the actual ratio were 1:2, then the larger number of reactants would be used up to maintain the ratio of reactants and to leave the smaller number of reactants left. Since all of 2 would be used up (33% less than 3), 33% of 1 would be used up (leaving 2/3 of the reactant left). The 2/3:2 is equivalent to a 1:3 ratio since 2/3 is 1/3 of 2. By analyzing the changing ratios from the original ratio of compounds to the actual compounds, you can find the excess ingredient as well as the limited reactant.
The above steps are how you solve simple stoichiometry problems. But, complicated ones may involve converting x g of this element to moles. In order to do so, you need to know the molar mass of the element, which can be found on the Periodic Table of Elements, hence making the Periodic Table of Elements quite useful. If you don't see molar mass in the squares, look for atomic mass because the two mean the same thing. The only difference is that atomic mass isn't necessarily attached to moles. It just determines how much mass an element contains. But, the molar mass is associated with the atomic mass per mole (6.02 * 10^23 particles).
Once you have found the molar mass, you can set up a proportion for the molar mass and the given mass of the element. Or, you could divide or multiply the given mass by the molar mass to find the number of moles depending on if the given mass greater than or less than the molar mass. If less than the molar mass, then it must be less than one mole, so you would simply divide the given mass by the molar mass. But, if the given mass is greater than the molar mass, then the molar mass must be divided by the given mass to find the number of moles.
Another complicated process of stoichiometry is if the compound contained more than one element. In this case, add up all the atomic masses together to find the molar mass. Another point to bring up is that molar mass can represent the total atomic mass of the compound whereas the atomic mass represents only one element.
One thing that wasn't mentioned was that stoichiometry only calculates the yield that could be formed. Theoretically speaking, for example, if an experiment involved combining a certain amount of sodium chloride with a certain amount of aluminum sulfate but you have a limited or excess amount of a compound, this will affect the yield you will produce. For example, if you could theoretically produce thrice the amount of sodium sulfate to twice the amount of aluminum chloride using 6NaCl + Al^2(SO4)3 ------- 3Na2(SO4) + 2Al(Cl3) but produced only 200g of sodium sulfate instead of the required 300g, then the yield is about 67%, or a ratio of 2:3. The yield percentage between moles and grams is proportional.
 |
| The yield percentage between moles and grams is proportional. |
Sunday, May 5, 2013
Mar. 29-Apr.3
This week, I learned about stoichiometry. This is the ratio of chemical reactants and products to one and another. For example, if 2NaCl + CO2 form 2(Na2)O and 1C(Cl2), then the ratios for NaCl to CO2 are 1:1, the ratios for CO2 to (Na2)O are 1:2, etc.
Anyone can say that 1 CO2 + 2 NaCl mean that there is one mole of carbon dioxide while there are two moles of sodium chloride. This, however, can only be true if the molar mass is equivalent to the number of moles (e.g. 1 mole oxygen=16 g). Therefore, it is possible for compounds to have different molar masses, hence number of moles as well.
A good example of this would be with 140 g of CO2 from the above chemical equation. For every 140g, how many moles of CO2 are there? To calculate the molar mass per mole, you must add the atomic masses of each element to each other. Knowing that diatomic oxygen has atomic mass of 32 (16*2) and that carbon has a molar mass of 12.11, you combine 32g to 12.11g to form 44.11 g/mole. But, as stated above, for every 140g of CO2, how many moles are there? To answer this question, set up the molar mass to 140g/X moles as a proportion. The answer is 3.17 moles of CO2. The steps are below:
But, this was, of course, is what we already know. This is just review. Now, let's move on to stoichiometry. In order to represent stoichiometry, we need to know the ratios first in the chemical reaction. Otherwise, if you don't balance the equation, you won't know the ratios, making it only harder to use stoichiometry. Even though it would be possible to still calculate the molar mass of the compounds, you wouldn't be able to identify the quantity of moles.
The next step after balancing the chemical equation is to calculate the molar mass of one of the reactants and find the moles of the other reactant and the products based on the ratios. In order to do so, represent this process using the BCA chart (bottom of the page).
In the problem above, propane is combined with oxygen gas. They yield the products of carbon dioxide and water. To balance the equation, look at the number of carbon in CO2 and propane. In propane, there are three, and in CO2, there is one. In order for this ratio to change from 3:1 to 3:3, there must be 3 CO2. The next step is to look at the hydrogen in propane and water. In propane, there are eight, and in water, there are 4 diatomic hydrogens. In order to keep the same number of hydrogens represented in the formulas and knowing water has diatomic hydrogen, there must be four diatomic hydrogen in order to have the same number of atoms as H8. Since the number of propane didn't have to change, there is only one molecule of propane. With 4 H2, there are four molecules of water. With three carbons, there are three molecules of carbon dioxide.
With one propane and five diatomic oxygen forming three carbon dioxide and four water, we can find the ratios.
However, the last part of the equation to balance is O2. In order to so, count the number of oxygen in water and carbon dioxide and combine them. Since there are three diatomic oxygen in carbon dioxide and 2 O2 (4 O=2 O2), there must have been five diatomic oxygen molecules to start out with.
The last step then is to do the calculations. Since it has been defined that there are 4.17 moles of propane, the moles of the other compounds is in sync with the ratio between propane and the other compounds. For example, propane to 5 O2 is 4.17 to 20.85 (4.17 * 5).
This is my favorite part. Under the before column, write down the number of moles the reactants start out. Then, for the products, write out 0 moles because the reactants haven't chemically combined through a combustion reaction yet. During the change column, this is the change in the number of moles from before to after (e.g. -4.17 moles of propane to represent a loss of moles on the reactant side). Since the moles are combining to form different compounds, the moles of the reactants would be zero in the after column since all the elements were used up with none to spare.
Then, for the products in the change, write the same number, except positive since it gained those moles, not lost them. In the after column, write the number of moles of the products.
Anyone can say that 1 CO2 + 2 NaCl mean that there is one mole of carbon dioxide while there are two moles of sodium chloride. This, however, can only be true if the molar mass is equivalent to the number of moles (e.g. 1 mole oxygen=16 g). Therefore, it is possible for compounds to have different molar masses, hence number of moles as well.
But, this was, of course, is what we already know. This is just review. Now, let's move on to stoichiometry. In order to represent stoichiometry, we need to know the ratios first in the chemical reaction. Otherwise, if you don't balance the equation, you won't know the ratios, making it only harder to use stoichiometry. Even though it would be possible to still calculate the molar mass of the compounds, you wouldn't be able to identify the quantity of moles.
The next step after balancing the chemical equation is to calculate the molar mass of one of the reactants and find the moles of the other reactant and the products based on the ratios. In order to do so, represent this process using the BCA chart (bottom of the page).
In the problem above, propane is combined with oxygen gas. They yield the products of carbon dioxide and water. To balance the equation, look at the number of carbon in CO2 and propane. In propane, there are three, and in CO2, there is one. In order for this ratio to change from 3:1 to 3:3, there must be 3 CO2. The next step is to look at the hydrogen in propane and water. In propane, there are eight, and in water, there are 4 diatomic hydrogens. In order to keep the same number of hydrogens represented in the formulas and knowing water has diatomic hydrogen, there must be four diatomic hydrogen in order to have the same number of atoms as H8. Since the number of propane didn't have to change, there is only one molecule of propane. With 4 H2, there are four molecules of water. With three carbons, there are three molecules of carbon dioxide.
With one propane and five diatomic oxygen forming three carbon dioxide and four water, we can find the ratios.
However, the last part of the equation to balance is O2. In order to so, count the number of oxygen in water and carbon dioxide and combine them. Since there are three diatomic oxygen in carbon dioxide and 2 O2 (4 O=2 O2), there must have been five diatomic oxygen molecules to start out with.
The last step then is to do the calculations. Since it has been defined that there are 4.17 moles of propane, the moles of the other compounds is in sync with the ratio between propane and the other compounds. For example, propane to 5 O2 is 4.17 to 20.85 (4.17 * 5).
This is my favorite part. Under the before column, write down the number of moles the reactants start out. Then, for the products, write out 0 moles because the reactants haven't chemically combined through a combustion reaction yet. During the change column, this is the change in the number of moles from before to after (e.g. -4.17 moles of propane to represent a loss of moles on the reactant side). Since the moles are combining to form different compounds, the moles of the reactants would be zero in the after column since all the elements were used up with none to spare.
Then, for the products in the change, write the same number, except positive since it gained those moles, not lost them. In the after column, write the number of moles of the products.
Sunday, April 28, 2013
Mar. 22-26
This week, I learned about the various ways ionic compounds could form.
The first type of reaction I learned about is a single
replacement reaction, which forms when the single reactant swaps place with one other same kind of element (e.g. AlN + P ––––> AlP + N), hence the products are an ionic compound and an element. But, if N were not singular in the reactant, then it as a diatomic atom, can bond to itself to form N2. But, once a product, the amount of nitrogen remains the same, so 2 nitrogens.
| Example of single replacement reaction |
Next, I learned about double replacement reactions.
These form when two double element compounds swap two elements into the other compound. This is how compounds, such as MgF2 and NaCl form. MgCl2 + NaF ––––> MgF2 +
NaCl is a perfect example of this. This forms a perfect product because the two
still are ionic compounds with 1) metal + nonmetal 2) Have neutral
charges. Double replacement reactions are like single replacement reactions because both involve ionic compounds, and sometimes, both have diatomic elements that bond to themselves at times to balance the
equation. But, the difference is that there are two ionic compounds in this reaction, not one.
Then, I learned about combustion reactions,
which is formed when a hydrocarbon and an oxygen always form the products of
carbon dioxide and water (e.g C3H8 + O2 ––––> CO2 +
H2O). Last week, I wrote that I wasn't sure as to how these combine to form CO2 and H2O. This reminds me of when I was learning about the process of photosynthesis in my Honors Biology course. All I knew was that glucose (C6H12O6) and O2 combined to form CO2 and H2O, but I wasn't sure as to what happened with the O5, the H10, and the C5. Essentially, CO2 and H2O are the products that form, but in biology, the quantity isn't labeled, which confuses many students.
 |
| Example of combustion reaction–photosynthesis |
To balance any chemical equation such as this one, glucose and diatomic oxygen, you need to look at the number of carbons first. Since there are 6, you can conclude that 6 CO2 will form. In the formula glucose itself, you can see the unsimplified empirical formula of water. With H12O6 there, it can be simplified to H2O since 12:6 has a 2:1 ratio, just as hydrogen does to oxygen. Therefore, since there are 12 H and H2O results, there are 6 H2O since H2=2 hydrogen atoms combined. Hence, with 6 H2, it stays proportionate with the 12 Hydrogens in one molecule of glucose.
But, to balance the reactants, look at the products. Since there are 6 O atoms in 6 H2O and 12 O atoms in 6 CO2, there is a total of 9 O2 since 6 O atoms in water is equivalent to 3 O2. So, to balance glucose and diatomic oxygen, there is 1 C6H12O6 and 6 O2 since the 1 O6 is equivalent to 3 O2.
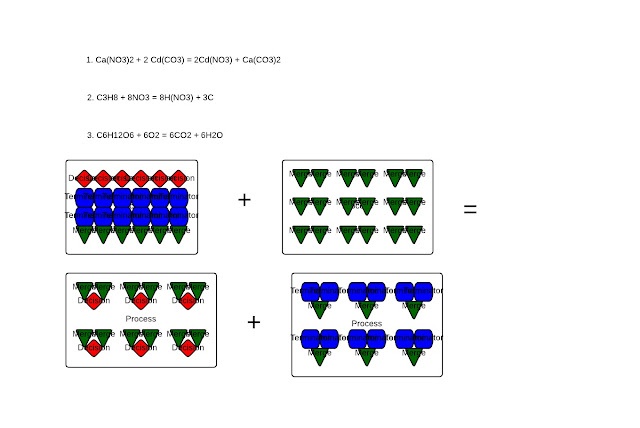 |
| Showing balanced equations and a particle diagram of photosynthesis |
Lastly, I learned about Synthesis and
Decomposition. Synthesis forms when two diatomic reactants combine to form a
single product (e.g. Na + I2 ––––> NaI).
Decomposition, on the other hand, is the inverted process of this. The single
reactant chemically separates (decomposes) into two elements, whether one of
them is diatomic or not (e.g. Na2(CO3) + 2HCl ––––> 2NaCl + H2(CO3)).
But, what does it mean to have 2 Li2(SO4) and 1 Al(PO4)? It means the ratio of the amount of each compound to the other in terms of moles. So, in other words, 2 LiNa means 2 moles of {Li and SO4} because 2[Li(SO4)] means 2Li and (SO4)2, and 1 Al(PO4) means 1 mole of {Al and PO4}. Hence, these moles have a 2:1 ratio.
In order to find their molar masses, one must find the molar masses of each element first, then look at the number before the compound to determine the number of moles, and to add the numbers together. But, before the molar masses of each element are listed, let's consider a very important rule: whatever the number of moles, ignore for now so you don't screw up your math. Once it's calculated, multiply by number before the compound.
For Li2(SO4), the molar mass of Li is 6.94, the molar mass of S is 32.07, and the molar mass of O is 16. But, to represent how they combined together, let's consider the number of the elements. Since Li2 is 2*{6.94 g/mole} + Sulfur + {32.07g/mole}+ 4 O *{16g/mole} form 109.95g/mole, we can conclude that the total molar mass of 2Li(SO4) is 219.9g.
But, what if you wanted to find the number of particles this compound has? To do so, find the molar mass first. In this case, we did, so we can multiply that by the number of particles for every mole, which is 6.02*10^23. Hence, the number of particles total is 1.32*10^26 particles.
Sunday, April 21, 2013
Mar 15-19
This week, I reviewed over binary and ternary ionic compounds, transition metals, molecular compounds, and acids. These were the topics I identified, defined, and charted in an Infographic I made on Lucidchart, which can be used through the Google Drive to make and store charts, graphs, and infographics.
The assignment on Monday was to define each of the following above and define how to find the chemical formulas of each. To make this, dragged the flowchart boxes onto the page from the menu on the left side. To type in information, click the Text icon and click in the box. This will frame the textbox, where you can type anything in. To resize the items, click on one of the diagonal corners and drag.
The neat thing about Lucidchart is that you don't have to worry about proportion size because it has a lock feature, which keeps the object in proportional size.
These are the things I learned as I made went along. I've never used Lucidchart before, but because of my tech curiosity and Mr. Abud's recommendation, I tried it out.
However, since I couldn't use this in class due to slow Internet connection, I used it on an up-to-date MacBook, which has great Intel and Internet connection, at home.
To make the infographic, I creatively chose different colors for the divisions of ionic compounds, then molecular compounds, acids, etc. I then used differently-shaped boxes to list examples of different compounds. I used relevant pictures for each of the chemical compounds and placed them underneath the charts, but I had to drag the pictures onto my desktop first and then upload them onto Lucidchart.
In the boxes, I defined binary compounds as simply a metal and a nonmetal chemically combining due to opposite charges and attractions. I then chose NaCl as one and said that to write the formulas of binary compounds, the charges must add up to zero because ionic compounds form neutral charges, and if a compound had a positive charge and a negative charge that wasn't the opposite of the positive charge (e.g. -2 and 1), then you multiply the lesser charges, or both if necessary, by the least common multiple. This, I noticed, was the same way to write the formulas with ternary compounds. Ternary compounds are formed when a polyatomic ion and a metal element (sometimes a transition metal) chemically combine due to ionic charges. An example of a ternary compound is Na(NO3). An example of a ternary compound with a transition metal is Cu III PO4 because copper, a positively charged transition metal, can change its charge depending on the negatively charged nonmetal. Hence, as PO4 loses three electrons to neutralize its own charge, it simultaneously neutralizes copper's charge by making it less positive.
I also then defined a molecular compound, which I said was formed when nonmetal elements combine due to covalent bonding (sharing of electrons). Based on the number of electrons shared, this defines their negative charge (e.g. NO3 has -1 charge because nitrogen has one less negatively-charged electron than oxygen, hence the charge is -1).
Lastly, I mentioned acids, which I said could be formed as long as hydrogen was written first in the formula and made the charges of the compound neutral (e.g. acetate acid is neutral because the C2H2O3 gives one electron to the hydrogen, hence making acetate more positive and the hydrogen less positive.) But, either way, acids are like ionic compounds because they both have neutral charges.
To convey this information, I linked arrows to the boxes to whatever boxes pertained to those boxes. But, this seems confusing alone without any sort of way to differentiate the information, which is why I used different colors. I colored the boxes pertaining to ionic compounds in general yellow, binary compounds green, ternary compounds blue, transition metal ternary compounds light teal, acids grey, and molecular compounds peach.
In case I missed any information or left a few things vague, I made a list of the rules for writing chemical formulas for ionic and molecular compounds on the side.
Once I completed my infographic and shared via Twitter and Google Drive, I also wrote a blog reflection. I think that writing blog reflections is a good way to look at how other people organize the information they think is important to discuss. However, since I am a perfectionist, I also find one of the cons of reading other blogs to be that some don't put in the effort, some leave gaps in information, or some blog posts have not been posted. But, by looking at both the pros and cons, I can fairly assess other people's blogs and grade them based on the requirements they met, or lack thereof.
However, I am also hypersensitive to criticism. Since I put in a great detail of attention to blogs and update whenever necessary, I hate it if other people rate me a 3.5, not a 4, which is the highest grade possible.
I also think that reading other blogs can be helpful if I need to study for assessments and have gaps in my knowledge on chemistry that I need to fill.
The first type of reaction I learned about is a single replacement reaction, which forms when the diatomic atoms (Bromine, Iodine, Nitrogen, Chlorine, Hydrogen, Oxygen, and Flourine) bond to themselves to form diatomic molecules (Br2, I2, etc) in order to form chemical compounds.
Next, I learned about double replacement reactions. Although this seems self-explanatory, I will explain. Think of four baskets of four fruits. Two of the baskets are in one group while the other two are in a different group. As soon as the traders decide to swap one of the baskets from each group, you get a different combination of fruits. This is how it works in order to form neutrally charged MgF2 + NaCl. MgCl2 + NaF ––––> MgF2 + NaCl is a perfect example of this. This forms a perfect product because the two still are ionic compounds wiht 1) metal + nonmetal 2) Have neutral charges. In a sense, these are like single replacement reactions because both may have diatomic elements that bond to themselves at times to balance the equation.
Then, I learned about combustion reactions, which are one of the easiest of compounds to remember because the reactants of hydrocarbon and an oxygen always form the products of carbon dioxide and water (e.g C3H8 + O2 ––––> CO2 + H2O). Although they combine to form neutrally-charged compounds, I'm not sure as to why C3H8 was combined the way it was without being neutrally charged and what happened to the remaining C2, H7, and how did the extra oxygen form from analready diatomic oxygen? Did it have to do with the fact that H2 + O2 ––––> H2O, and the extra O combined with C3H7?
Lastly, I learned about Synthesis and Decomposition. Synthesis forms when two diatomic reactants combine to form a single product (e.g. H2 + O2 ––––> H2O). Decomposition, on the other hand, is the inverted process of this. The single reactant chemically separates (decomposes) into two elements, whether one of them is diatomic or not (e.g. NaCl ––––> Na + diatomic Cl2). I, however, am not sure as to how a compound can decompose, and I want to know the chemistry behind it. I hypothesize that it may have to do with the fact that an electrical current can pass through and separate them, like when H2O ––––> H2 + O2 form during the electrolysis experiment.
The assignment on Monday was to define each of the following above and define how to find the chemical formulas of each. To make this, dragged the flowchart boxes onto the page from the menu on the left side. To type in information, click the Text icon and click in the box. This will frame the textbox, where you can type anything in. To resize the items, click on one of the diagonal corners and drag.
The neat thing about Lucidchart is that you don't have to worry about proportion size because it has a lock feature, which keeps the object in proportional size.
These are the things I learned as I made went along. I've never used Lucidchart before, but because of my tech curiosity and Mr. Abud's recommendation, I tried it out.
However, since I couldn't use this in class due to slow Internet connection, I used it on an up-to-date MacBook, which has great Intel and Internet connection, at home.
 |
| The picture shows the menu options from top to bottom: 1) Text and standard boxes 2) Flowcharts 3) Containers 4) Shapes 5) Upload Image |
In the boxes, I defined binary compounds as simply a metal and a nonmetal chemically combining due to opposite charges and attractions. I then chose NaCl as one and said that to write the formulas of binary compounds, the charges must add up to zero because ionic compounds form neutral charges, and if a compound had a positive charge and a negative charge that wasn't the opposite of the positive charge (e.g. -2 and 1), then you multiply the lesser charges, or both if necessary, by the least common multiple. This, I noticed, was the same way to write the formulas with ternary compounds. Ternary compounds are formed when a polyatomic ion and a metal element (sometimes a transition metal) chemically combine due to ionic charges. An example of a ternary compound is Na(NO3). An example of a ternary compound with a transition metal is Cu III PO4 because copper, a positively charged transition metal, can change its charge depending on the negatively charged nonmetal. Hence, as PO4 loses three electrons to neutralize its own charge, it simultaneously neutralizes copper's charge by making it less positive.
I also then defined a molecular compound, which I said was formed when nonmetal elements combine due to covalent bonding (sharing of electrons). Based on the number of electrons shared, this defines their negative charge (e.g. NO3 has -1 charge because nitrogen has one less negatively-charged electron than oxygen, hence the charge is -1).
Lastly, I mentioned acids, which I said could be formed as long as hydrogen was written first in the formula and made the charges of the compound neutral (e.g. acetate acid is neutral because the C2H2O3 gives one electron to the hydrogen, hence making acetate more positive and the hydrogen less positive.) But, either way, acids are like ionic compounds because they both have neutral charges.
To convey this information, I linked arrows to the boxes to whatever boxes pertained to those boxes. But, this seems confusing alone without any sort of way to differentiate the information, which is why I used different colors. I colored the boxes pertaining to ionic compounds in general yellow, binary compounds green, ternary compounds blue, transition metal ternary compounds light teal, acids grey, and molecular compounds peach.
In case I missed any information or left a few things vague, I made a list of the rules for writing chemical formulas for ionic and molecular compounds on the side.
 |
| These icons are ways to organize information. |
However, I am also hypersensitive to criticism. Since I put in a great detail of attention to blogs and update whenever necessary, I hate it if other people rate me a 3.5, not a 4, which is the highest grade possible.
I also think that reading other blogs can be helpful if I need to study for assessments and have gaps in my knowledge on chemistry that I need to fill.
The Various Ways Chemical Compounds Form …
Lastly, I learned further about writing chemical formulas and ascertaining what compounds will form after chemical reactions. As long as one knows the types of compounds that can be formed, it comes easily–as it did for me. |
| Single replacement reaction |
The first type of reaction I learned about is a single replacement reaction, which forms when the diatomic atoms (Bromine, Iodine, Nitrogen, Chlorine, Hydrogen, Oxygen, and Flourine) bond to themselves to form diatomic molecules (Br2, I2, etc) in order to form chemical compounds.
Next, I learned about double replacement reactions. Although this seems self-explanatory, I will explain. Think of four baskets of four fruits. Two of the baskets are in one group while the other two are in a different group. As soon as the traders decide to swap one of the baskets from each group, you get a different combination of fruits. This is how it works in order to form neutrally charged MgF2 + NaCl. MgCl2 + NaF ––––> MgF2 + NaCl is a perfect example of this. This forms a perfect product because the two still are ionic compounds wiht 1) metal + nonmetal 2) Have neutral charges. In a sense, these are like single replacement reactions because both may have diatomic elements that bond to themselves at times to balance the equation.
Then, I learned about combustion reactions, which are one of the easiest of compounds to remember because the reactants of hydrocarbon and an oxygen always form the products of carbon dioxide and water (e.g C3H8 + O2 ––––> CO2 + H2O). Although they combine to form neutrally-charged compounds, I'm not sure as to why C3H8 was combined the way it was without being neutrally charged and what happened to the remaining C2, H7, and how did the extra oxygen form from analready diatomic oxygen? Did it have to do with the fact that H2 + O2 ––––> H2O, and the extra O combined with C3H7?
Lastly, I learned about Synthesis and Decomposition. Synthesis forms when two diatomic reactants combine to form a single product (e.g. H2 + O2 ––––> H2O). Decomposition, on the other hand, is the inverted process of this. The single reactant chemically separates (decomposes) into two elements, whether one of them is diatomic or not (e.g. NaCl ––––> Na + diatomic Cl2). I, however, am not sure as to how a compound can decompose, and I want to know the chemistry behind it. I hypothesize that it may have to do with the fact that an electrical current can pass through and separate them, like when H2O ––––> H2 + O2 form during the electrolysis experiment.
Therefore, by understanding the different ways compounds form and decompose, one can get the basic understanding that this is how ionic, molecular, and acidic compounds form.
 |
| Synthesis reaction |
Sunday, April 14, 2013
Mar 8-12
This week, I learned about molecular compounds through the practice problems I did in class.
A molecular compound is formed when two or more nonmetal elements combine to form a compound. They attract through sharing, not transferring electrons. This then differentiates between ionic and nonionic compounds.
The differences between an ionic and a molecular compound are that an ionic is form by metal and nonmetal elements that attract to each other because of their opposite charges (+ metal and - nonmetal) and transferring of electrons to neutralize their charges. On the other hand, a molecular compound is form when the nonmetal elements play tug of war with electrons but don't transfer them. Hence, molecular compounds don't have neutral charges. They are negatively charged for the most part.
Because molecular compounds don't share electrons, I realized that they bonded in different ways. They share electrons through covalent bonding, which occurs when the negatives in atoms don't attract due to the negative nonmetal elements. Instead, they repel and reveal the positives. This then creates an overlapping area of electrons. The electrons are shared between the two atoms combined. The mobile electrons in the molecular compound rotate anywhere, but mainly near the more slightly positive atom due to a weak attraction between the electrons and the atom. But, the electrons, when they approach the other electrons, repel, due to the same negative charges.
This week, I also learned about how to combine molecular compounds and ionic compounds as well. To combine ionic compounds, the ions must neutralize in order to form an ionic compound. One of the board problems I did involved combining a "polyatomic ion" (a negatively charged conglomeration of many atoms) with a transition metal. To solve those, I knew I had to know the charge of the polyatomic ion first since the transition metal's charge varies depending on the charge of the other element. This is because an ionic bond attracts the transition metal and the nonmetal polyatomic ion together, forming a neutrally charged ionic compound.
For example, in Cu(NO4), the charge of copper would be +1 since the nitrate has a charge of -1. As the copper combines with the nitrate, the nitrate transfers electrons to copper, neutralizing copper and nitrate. With the needed electron in NO4, not only does the charge become neutral, but I also think that an ionic bond now holds N and O4 together, making their attraction to each other even stronger. Therefore, the new ionic compound formed is Copper (I) Nitrate. The Roman numeral indicates a transition metal's charge.
I also wondered about labeling chemical compounds if the subscript of each was a certain number. If I had to label the specific compound, what would I call it? For example, what would I call N5O2? Knowing that there are 5 nitrogen atoms and 2 oxygen atoms, this compound is called penta-nitrogen dioxide. Therefore, I learned that depending on the subscript of the elements, you use the prefix (tetra, for example, to represent four) and put it before the element. The second or last element in the compound has the suffix -ide at the end, except with hydrogen.
One of the baffling things also seemed to center around the polarity between H2O. I am not sure as to why, but oxygen has an uneven pull on the hydrogen atoms. But, how can this be when they have a neutral charge? It seems to me that polar substances only form if the compound isn't neutrally charged. Therefore, I think it's possible for molecular compounds to have polarity.
In H2O, the electrons seem to attract to one other H atom, making it more negative, and the other one more positive. Knowing that the electrons repel each other and are mobile, they can move around the more positive H atom. But, for the most part, the electrons surround the other H atom. Because of this, I wonder if this may be linked to or causes its polarity.
I also wonder if two nonmetal elements combine and have a neural charge, then would they form an ionic compound or a molecular compound. An example I can think of this is C(NO3)4 because carbon has +4 charge and (NO3)4 with a charge of -4, hence forming a neutral charge. This I seemed to struggled with because I like to think that there is always a grey area in between and that things aren't just black and white. I think C(NO4)4 is an ionic compound because carbon has a charge of +4 and NO4 has a charge of -1, so C(NO4)4, logically, would neutralize and become ionic through ionic bonding. But, then C(NO4)4 could be a molecular compound because carbon and nitrate are both nonmetals and carbon may transfer or receive electrons depending on other atoms it combines with. Finding more support for the latter choice, I think it's most likely a molecular compound since both are nonmetals.
A molecular compound is formed when two or more nonmetal elements combine to form a compound. They attract through sharing, not transferring electrons. This then differentiates between ionic and nonionic compounds.
 |
| Ionic bonding=transferring and receiving of electrons |
The differences between an ionic and a molecular compound are that an ionic is form by metal and nonmetal elements that attract to each other because of their opposite charges (+ metal and - nonmetal) and transferring of electrons to neutralize their charges. On the other hand, a molecular compound is form when the nonmetal elements play tug of war with electrons but don't transfer them. Hence, molecular compounds don't have neutral charges. They are negatively charged for the most part.
Because molecular compounds don't share electrons, I realized that they bonded in different ways. They share electrons through covalent bonding, which occurs when the negatives in atoms don't attract due to the negative nonmetal elements. Instead, they repel and reveal the positives. This then creates an overlapping area of electrons. The electrons are shared between the two atoms combined. The mobile electrons in the molecular compound rotate anywhere, but mainly near the more slightly positive atom due to a weak attraction between the electrons and the atom. But, the electrons, when they approach the other electrons, repel, due to the same negative charges.
This week, I also learned about how to combine molecular compounds and ionic compounds as well. To combine ionic compounds, the ions must neutralize in order to form an ionic compound. One of the board problems I did involved combining a "polyatomic ion" (a negatively charged conglomeration of many atoms) with a transition metal. To solve those, I knew I had to know the charge of the polyatomic ion first since the transition metal's charge varies depending on the charge of the other element. This is because an ionic bond attracts the transition metal and the nonmetal polyatomic ion together, forming a neutrally charged ionic compound.
-nitrate-trihydrate-sample.jpg) |
| Copper I Nitrate |
I also wondered about labeling chemical compounds if the subscript of each was a certain number. If I had to label the specific compound, what would I call it? For example, what would I call N5O2? Knowing that there are 5 nitrogen atoms and 2 oxygen atoms, this compound is called penta-nitrogen dioxide. Therefore, I learned that depending on the subscript of the elements, you use the prefix (tetra, for example, to represent four) and put it before the element. The second or last element in the compound has the suffix -ide at the end, except with hydrogen.
 |
| Covalent bonding=sharing of electrons |
In H2O, the electrons seem to attract to one other H atom, making it more negative, and the other one more positive. Knowing that the electrons repel each other and are mobile, they can move around the more positive H atom. But, for the most part, the electrons surround the other H atom. Because of this, I wonder if this may be linked to or causes its polarity.
I also wonder if two nonmetal elements combine and have a neural charge, then would they form an ionic compound or a molecular compound. An example I can think of this is C(NO3)4 because carbon has +4 charge and (NO3)4 with a charge of -4, hence forming a neutral charge. This I seemed to struggled with because I like to think that there is always a grey area in between and that things aren't just black and white. I think C(NO4)4 is an ionic compound because carbon has a charge of +4 and NO4 has a charge of -1, so C(NO4)4, logically, would neutralize and become ionic through ionic bonding. But, then C(NO4)4 could be a molecular compound because carbon and nitrate are both nonmetals and carbon may transfer or receive electrons depending on other atoms it combines with. Finding more support for the latter choice, I think it's most likely a molecular compound since both are nonmetals.
Sunday, April 7, 2013
Mar. 25-28
 For the most part, the charges of the elements were consistent and unchanging. But, I realized that wasn't the case with transition metals. Transition metals, depending on the charges of the other elements they combine with to form an ionic compound, can alter its charge.
For the most part, the charges of the elements were consistent and unchanging. But, I realized that wasn't the case with transition metals. Transition metals, depending on the charges of the other elements they combine with to form an ionic compound, can alter its charge.During the beginning of the lab, CuCl was an ionic compound. Cl in CuCl has a charge of -1, hence Cu must have a charge of +1.
This is what I learned in the copper chloride lab. The objective was to see what would happen if water and copper chloride chemically combined together.
I hypothesized that when they combined, the copper would stay the same since it is a metal while chlorine, hydrogen, and oxygen are gases. To combine them together, we used the electrolysis equipment. My group and I filled the trough with 100 mL of water and 4 g of copper chloride.As the copper chloride was combined with the water, the water turned blue. I concluded this was the case because the copper oxidized as the CuCl bonded with H2O.
 But, if H2^2 charge + O^-2 charge + Cu^X charge + Cl^-1 charge combined, then how would they form an ionic compound? In order for the ionic compound to be neutral, the charge of copper would have to be +1. However, I noticed that at the bottom of the trough, there was oxidized copper. While the CuCl combined with water, the oxygen separated the copper while the H2 separated the chlorine. As the separation between the two occurred, the copper was oxidized by the oxygen atom in the process.
But, if H2^2 charge + O^-2 charge + Cu^X charge + Cl^-1 charge combined, then how would they form an ionic compound? In order for the ionic compound to be neutral, the charge of copper would have to be +1. However, I noticed that at the bottom of the trough, there was oxidized copper. While the CuCl combined with water, the oxygen separated the copper while the H2 separated the chlorine. As the separation between the two occurred, the copper was oxidized by the oxygen atom in the process.But, what would copper's charge be if it combined with oxygen? Knowing that copper is a conductive positively charged metal and knowing that oxygen has a charge of -2, copper combined with oxygen must have a charge of +2. In this case, then how would the chlorine combine with the H2O. Already knowing that H2O is an ionic compound already, it wouldn't make sense for it to combine with chlorine. Therefore, the copper and the chlorine were separated from each other as the copper chloride was combined with water.
 Because the CuCl dissolved in water, I concluded that the CuCl ions became polarized, hence interrupting intermolecular forces and phase changes. If this were the case, then I wonder if factors, such as melting point and freezing point would be different from CuCl and just copper and chlorine. If this would be true, then the attraction between the particles would change. I think that as copper and chloride were separated from the water, the attraction between the CuCl ions and the H2O ions was less than that of the attraction between copper and chloride atoms.
Because the CuCl dissolved in water, I concluded that the CuCl ions became polarized, hence interrupting intermolecular forces and phase changes. If this were the case, then I wonder if factors, such as melting point and freezing point would be different from CuCl and just copper and chlorine. If this would be true, then the attraction between the particles would change. I think that as copper and chloride were separated from the water, the attraction between the CuCl ions and the H2O ions was less than that of the attraction between copper and chloride atoms.Knowing that single elements regain their charge once separated from ionic compounds, I concluded this is what happened to chlorine and copper. Knowing that chlorine gain the electrons that copper lost, chlorine would become more positive while the copper became more negative.
 This week, I also participated in the electroscope lab, which pertains to charge induction. I made my electroscope using aluminum, a glass jar, and a plastic lid taped to the glass jar. To make the electroscope, I poked a hole in the middle of the ball and stuck the rod through it so the ball wouldn't fall off of the rod. Then I poked a hole in the plastic lid and stuck the rod through the hole. Then, I bent the bottom at a 90º angle and attached the unwrinkled and even-length aluminum leaves.
This week, I also participated in the electroscope lab, which pertains to charge induction. I made my electroscope using aluminum, a glass jar, and a plastic lid taped to the glass jar. To make the electroscope, I poked a hole in the middle of the ball and stuck the rod through it so the ball wouldn't fall off of the rod. Then I poked a hole in the plastic lid and stuck the rod through the hole. Then, I bent the bottom at a 90º angle and attached the unwrinkled and even-length aluminum leaves.Then, I tapped the rod to the plastic lid and then taped the lid on the glass charge.
To test the conductivity of my electroscope, I rubbed a balloon against my shirt to charge the balloon. As soon as I held the balloon over the aluminum ball, electrostatic electricity took place in the aluminum rod. I almost made the mistake that the electrons were being transferred from the balloon to the aluminum. But, as soon as I differentiated between that and chemically combined substances, I then realized that the polarity was only being affected, not the number of electrons between the balloon and the aluminum.
 Since the aluminum itself was neutrally charged just as the balloon was, charge induction occurred. The negative charges in the aluminum were only being shifted as the balloon was brought near it. Knowing that the electrical current passed through the rod, I concluded that it must have passed through the leaves at the bottom. Since the polarity was shifted, I figured that the negatives and positives were being evenly shifted together toward the leaves.
Since the aluminum itself was neutrally charged just as the balloon was, charge induction occurred. The negative charges in the aluminum were only being shifted as the balloon was brought near it. Knowing that the electrical current passed through the rod, I concluded that it must have passed through the leaves at the bottom. Since the polarity was shifted, I figured that the negatives and positives were being evenly shifted together toward the leaves.
Sunday, March 24, 2013
Mar. 18-22
But, I then wondered: Is it possible to have an equal amount but to not be evenly distributed? Indeed, it is so. This state is called polarization.
This is what I was thinking when the class was given an in-class demonstration. The sweater and the balloon initially didn't attract each other because they had neutral charges. But, that changed when the balloon was rubbed against the wall, it became more positive while the wall became negative. Using my prior knowledge, I assumed that this happened because I thought that as electrons were being transferred from the balloon to the wall, the wall became more negative while the balloon became more positive.
 But, how did the sweater attract to the balloon? How did it become negative? Doesn't this only apply to things with a positive charge while the other has a negative charge? It turns out that both went through charge induction, which I at first thought it was when opposite charges were brought together through neutral charges. But, what charge induction really is, is when one object is charged near a neutral charge and changes the distribution of positive and negative ions in order to create two polar ends.
But, how did the sweater attract to the balloon? How did it become negative? Doesn't this only apply to things with a positive charge while the other has a negative charge? It turns out that both went through charge induction, which I at first thought it was when opposite charges were brought together through neutral charges. But, what charge induction really is, is when one object is charged near a neutral charge and changes the distribution of positive and negative ions in order to create two polar ends.Essentially, the balloon was moved to the wall and nothing happened initially since both were neutral to start with. Then, when it was moved to the sweater and back to the wall, it polarizes the wall shifting the negative ions from the positive ions.
I then wondered why the negative ions were moved rather than the positive ions. Did it have to do with the greater electrostatic strength of positive ions than negative ions? Or was it because the opposite charges of positive ions and negative ions held each other in place? It seems as if though this is the case because I think that the positive ions are stronger than the negative ions. I theorize, although most likely erroneously, that the electrostatic strength has an inverse relationship with the number of electrons. It seems as if though the negative ions have less strength because they have a greater number of electrons than the positive ions. The reason I think this is so is because I think that the less electrons there are, the less pressure is being put on the "electro-chain" that holds the electrons in place. Hence, the charge of positive ions is stronger than that of negative ions.
I then wonder if this could play into the particle attraction between solids, liquids, and gases. It seems like the positive and negative ions lock the location of the particles based on their charges, hence bringing the molecules together. This may be influencing the decreasing ph and th energy in a liquid, hence slowing the the particles and decreasing the amount of space they move and turning to solid as the particles form a strongly attracted lattice structure.
A liquid on the other hand, has less attraction between the particles than the solid, so they move about more freely. I think this may be because they have some attraction but mainly repel. I think they partially attract because if they didn't when a liquid is in a container, the particles in the liquid would still move farther apart. But, because there is less space to spread out, the particles don't move apart as much.
A gas, on the other hand, seems to repel even more than a liquid and a solid, with little or no degree of attraction. If a gas were not in a container, the particles would move freely due to lack of attraction, which would explain why pollutants in the air can spread out so much in the atmosphere, aside from the winds. But, if a gas were in a container, the particles would have some degree of attraction but less than a liquid. There is a greater probability that gas particles could move apart from each other and then collide again because there is less space for the particles to move around in the container.
 Overall, substances can change state because of their changing electrostatic forces and attractions between particles at an atomic level.
Overall, substances can change state because of their changing electrostatic forces and attractions between particles at an atomic level.This same principle also applies to ionic compounds, which form when ions transfer electrons to form neutral compounds. Through the exchange of electrons, one element, of course, becomes positive while the other one becomes negative. This is what happens when NaCl combine. The Na needed to lose an electron while the Cl needed to gain one. So, through this "electron exchange", the Na became positive and the Cl became negative. They then formed an ionic compound, which means a neutral compound has formed through electron exchanges with ions.
Subscribe to:
Posts (Atom)























