This week, I learned about the various ways ionic compounds could form.
The first type of reaction I learned about is a single
replacement reaction, which forms when the single reactant swaps place with one other same kind of element (e.g. AlN + P ––––> AlP + N), hence the products are an ionic compound and an element. But, if N were not singular in the reactant, then it as a diatomic atom, can bond to itself to form N2. But, once a product, the amount of nitrogen remains the same, so 2 nitrogens.
| Example of single replacement reaction |
Next, I learned about double replacement reactions.
These form when two double element compounds swap two elements into the other compound. This is how compounds, such as MgF2 and NaCl form. MgCl2 + NaF ––––> MgF2 +
NaCl is a perfect example of this. This forms a perfect product because the two
still are ionic compounds with 1) metal + nonmetal 2) Have neutral
charges. Double replacement reactions are like single replacement reactions because both involve ionic compounds, and sometimes, both have diatomic elements that bond to themselves at times to balance the
equation. But, the difference is that there are two ionic compounds in this reaction, not one.
Then, I learned about combustion reactions,
which is formed when a hydrocarbon and an oxygen always form the products of
carbon dioxide and water (e.g C3H8 + O2 ––––> CO2 +
H2O). Last week, I wrote that I wasn't sure as to how these combine to form CO2 and H2O. This reminds me of when I was learning about the process of photosynthesis in my Honors Biology course. All I knew was that glucose (C6H12O6) and O2 combined to form CO2 and H2O, but I wasn't sure as to what happened with the O5, the H10, and the C5. Essentially, CO2 and H2O are the products that form, but in biology, the quantity isn't labeled, which confuses many students.
 |
| Example of combustion reaction–photosynthesis |
To balance any chemical equation such as this one, glucose and diatomic oxygen, you need to look at the number of carbons first. Since there are 6, you can conclude that 6 CO2 will form. In the formula glucose itself, you can see the unsimplified empirical formula of water. With H12O6 there, it can be simplified to H2O since 12:6 has a 2:1 ratio, just as hydrogen does to oxygen. Therefore, since there are 12 H and H2O results, there are 6 H2O since H2=2 hydrogen atoms combined. Hence, with 6 H2, it stays proportionate with the 12 Hydrogens in one molecule of glucose.
But, to balance the reactants, look at the products. Since there are 6 O atoms in 6 H2O and 12 O atoms in 6 CO2, there is a total of 9 O2 since 6 O atoms in water is equivalent to 3 O2. So, to balance glucose and diatomic oxygen, there is 1 C6H12O6 and 6 O2 since the 1 O6 is equivalent to 3 O2.
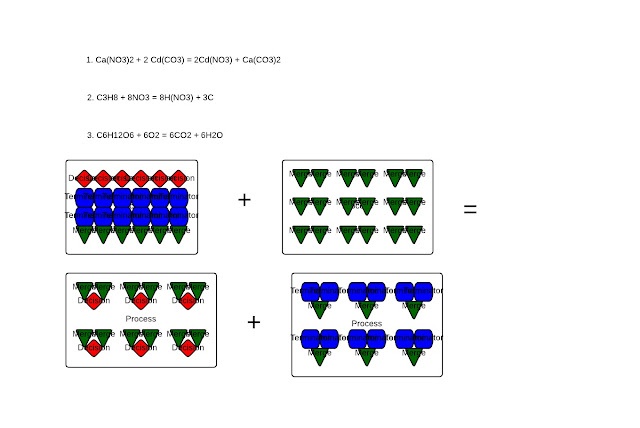 |
| Showing balanced equations and a particle diagram of photosynthesis |
Lastly, I learned about Synthesis and
Decomposition. Synthesis forms when two diatomic reactants combine to form a
single product (e.g. Na + I2 ––––> NaI).
Decomposition, on the other hand, is the inverted process of this. The single
reactant chemically separates (decomposes) into two elements, whether one of
them is diatomic or not (e.g. Na2(CO3) + 2HCl ––––> 2NaCl + H2(CO3)).
But, what does it mean to have 2 Li2(SO4) and 1 Al(PO4)? It means the ratio of the amount of each compound to the other in terms of moles. So, in other words, 2 LiNa means 2 moles of {Li and SO4} because 2[Li(SO4)] means 2Li and (SO4)2, and 1 Al(PO4) means 1 mole of {Al and PO4}. Hence, these moles have a 2:1 ratio.
In order to find their molar masses, one must find the molar masses of each element first, then look at the number before the compound to determine the number of moles, and to add the numbers together. But, before the molar masses of each element are listed, let's consider a very important rule: whatever the number of moles, ignore for now so you don't screw up your math. Once it's calculated, multiply by number before the compound.
For Li2(SO4), the molar mass of Li is 6.94, the molar mass of S is 32.07, and the molar mass of O is 16. But, to represent how they combined together, let's consider the number of the elements. Since Li2 is 2*{6.94 g/mole} + Sulfur + {32.07g/mole}+ 4 O *{16g/mole} form 109.95g/mole, we can conclude that the total molar mass of 2Li(SO4) is 219.9g.
But, what if you wanted to find the number of particles this compound has? To do so, find the molar mass first. In this case, we did, so we can multiply that by the number of particles for every mole, which is 6.02*10^23. Hence, the number of particles total is 1.32*10^26 particles.













-nitrate-trihydrate-sample.jpg)





