 On Tuesday of this week, we compared the mass, the volume, and most importantly, the density of the different liquids in our labs, which were fruit punch, apple juice, oil, water, Coca-Cola, Diet Coke, Sprite, and Dr. Pepper. My group and I measured Coca-Cola, so we measured 10 mL of it and then recorded it, then we also measured 20 mL, then 30 mL, then 40 mL, and lastly, 50 mL.
On Tuesday of this week, we compared the mass, the volume, and most importantly, the density of the different liquids in our labs, which were fruit punch, apple juice, oil, water, Coca-Cola, Diet Coke, Sprite, and Dr. Pepper. My group and I measured Coca-Cola, so we measured 10 mL of it and then recorded it, then we also measured 20 mL, then 30 mL, then 40 mL, and lastly, 50 mL.After the experiment, my group and I collected our data, and we posted our results on Lino because we ensured that our data was correct. To measure the density of Coca-Cola correctly, we excluded the mass of the graduation cylinder because we were just looking for the density of the liquid.
But, my intuition told me that most of the students forgot to omit the mass of the graduation cylinders. I was thinking what would happen next, which would be that all the groups would have to compare the densities of all the liquids, and my trepidation was that the overall masses and densities would be incorrect, so the data would be as well. Fortunately though, the group with the Diet Coke rectified their data since their density seemed too big, which made me feel less uncertain.
On Tuesday, I made a "revelation," as Mr. Abud termed it, about density. It sparked a memory stretching back from 7th grade. In 7th grade, I had to learn how to calculate the density of water, which was 1g/1cm^3. In a one-second flash, I though that density equaled the mass divided by the volume. So I had the courage to raise my hand and say what I was thinking as we were comparing all of the graphs of the densities of the liquids. I helped the students learn in a class discussion that d=m/v, and that d(v)=m are the density formulas, which my group and I used to help to calculate the density of Coca-Cola.
But the weirdest thing is that I was using it the whole time, and I just happened to have brainstormed the obvious! In retrospect, it was awesome because my group thought I was a total genius that day. But, the best part is that I even thought like a scientist that day and contributed to a class discussion in an ingenious way. It was definitely the best part of my day--and week.
 We also had a class discussion on density. For example, Mr. Abud demonstrated to the
whole class two products from our lab: water and vegetable oil. He asked us what would happen if you were to put them in a graduation cylinder. Everyone hypothesized that the oil would float on top of the water
based on our data from our experiment, which proved that oil was less
dense than water. Indeed, the oil did float on top of the water.
We also had a class discussion on density. For example, Mr. Abud demonstrated to the
whole class two products from our lab: water and vegetable oil. He asked us what would happen if you were to put them in a graduation cylinder. Everyone hypothesized that the oil would float on top of the water
based on our data from our experiment, which proved that oil was less
dense than water. Indeed, the oil did float on top of the water.Even though I learned what mass and volume were last week, this lab helped me review the difference between mass and volume, which is that mass is how much matter an object contains, and volume is how much space an object contains.
 I learned that density is the amount of matter in a certain amount
of space, which made perfectly logical, mathematical sense to me
because the amount of matter is the mass, and the amount of space
is the volume. In my opinion. the definition for density was
I learned that density is the amount of matter in a certain amount
of space, which made perfectly logical, mathematical sense to me
because the amount of matter is the mass, and the amount of space
is the volume. In my opinion. the definition for density wasjust the formula converted into words.
During this week, I learned that mass and volume were inversely related in terms of density. For example, if you
had an object with less mass and greater volume, then you
would have smaller density. Or, if you had greater mass and less volume, then you would have a larger density.
For this week, though, if I had to improve on one thing in order to succeed, I should relax and calm down because I tend to worry about what's going to happen next rather than focusing on the here and now, and then I would think of all the possibilities I would have to face head-on. I knew everything for the assessment and realized I didn't have to study much, except mentally review over what we did and learned this week and the week before. I speculated that I would get an A because I knew the material, but I was panicking for no reason, so my group members, Madison, Jaimie, and Margo told me to calm down. Even Mr. Abud was witnessing this, and he told us that everything was cool. In retrospect, they're right.
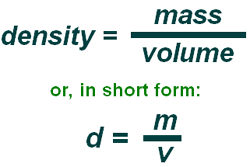
No comments:
Post a Comment