 |
| Hydrogen, although less dense, has twice as much volume as hydrogen as shown in the balloons. |
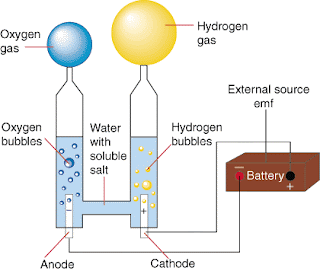
But, how could I find a way to prove this true? Well, this week, my group and I did an electrolysis experiment. We had to hook up two probes to two metals ends at the bottom of the trough. We had to fill the trough with sodium chloride water solution. But, why not water? This is the part I felt I wasn't so sure of, but I think it had to do with the fact that sodium chloride could conduct electricity in order for the hydrogen and oxygen particles to chemically split.
Then, what we had to do next was use two graduation cylinders (one for hydrogen and the other for oxygen) and put them on top of the metal ends making sure that they are still full. The tricky part was to do so and sort of break the rules of physics–in other words, have none of the tubes with the sodium chloride water solution gravitate down the tubes. To do, I learned this with trial and error. It then came to me after the experiment that it was like the gas-trough lab where the bottles had to be filled with water and put in the trough and still remain full. The only difference, which screwed me up, was imagining that I was using a lens to cover the top of the tube. So, I had to approach this differently. Scoop the tubes with the solution and slowly tilt them to the bottom of the trough, then tilt it again toward the metal end and cover it with the tube without going over the top of the tube.
 |
| Scientific notation review: a) With small numbers less than 1, the exponent is negative. b) With large numbers greater than 10, the exponent is positive. |
There was 3.5 mL of oxygen left in the tube. The question was to determine the molar mass of H2O by finding the molar masses of H2 and O through experimentation and mathematics:
To find x grams of oxygen for 3.5 mL:
3.5
---------- -------------
1
Twice as much volume as the oxygen, the volume of hydrogen left in the tube was 7 mL. Also, the same process was used for hydrogen in order to find the x grams of hydrogen per mL:
7 mL* 0.089g * 1 L
----------- ------------
1 L 1000 mL
The answer is 0.000623g, but by using scientific notation once again, the answer is 6.23*10^-4 g for one hydrogen.
Based on this lab, I have concluded that hydrogen is less dense than oxygen. Therefore, my hypothesis was correct. Doing more research on hydrogen and oxygen to further support my hypothesis, oxygen has a greater atomic mass (16) than that of hydrogen (1). The atomic mass ratio between 1 oxygen and 1 hydrogen, therefore, is 16-1. But, the ratio with 1 oxygen to 2 hydrogens, is 8-1. This proves that oxygen still has a greater density than hydrogen–just a double dip ratio from 16-1 to 8-1.
But, I still wonder what would have happened if the probes (the anobe and the cathobe) were hooked up differently? Would that have altered the results possibly skewing them, or left them the same? Keeping in mind that everyone turned the handle clockwise, what would have happened if we turned it counter-clockwise? Would this have affected which tubes the hydrogen and the oxygen would have gone into? It seems to me in the picture above that the anobe attracts oxygen and the cathobe attracts with hydrogen. If that is to be so, then I think it's possible that oxygen may have a negative charge while hydrogen may have a positive. I think that since there are two hydrogens and 1 oxygen with a 2-1 ratio when combined to make H2O, I think that oxygen has a -2 charge while the two hydrogens have a -1 charge each. This would further help to explain why the two can chemically combine to form a compound. Now that I think about it, the anobe (negative) and the cathobe (positive) if were flip-flopped may have the same impact on where the oxygen and the hydrogen go. Here's some thinking ahead: Through the electrolysis lab, I wonder if next week I will learn about how electrons may tie into electrolysis?

No comments:
Post a Comment