 |
| J.J. Thompson's Plum Pudding model |
Based on this, I realized that we would have to change the way we draw particle diagrams to represent them at the smallest level. Particle diagrams in the past units could still be drawn the same using dots to represent particles. But, to draw the new particle diagrams, I thought of two possible ways to draw them: 1) Draw a circle to represent a particle and inside the circle, use + to represent positive and – to represent negative. 2) Draw a circle and inside the circle, draw dots to represent the charge. The second option is how we draw them.
 Charges occur when the electrons transfer from one place to another. Depending on the charges, they could charge with other things. This is what I learned in the sticky tape lab. In this lab, we tested two pieces of tape: one tape taped onto another tape. The objective was to test the top tape and the bottom tape with other things to determine whether they would repel or attract. The top tape that was ripped off the bottom tape seemed to attract to the bottom tape. But, why? I at first thought that the top tape got charge after tearing it off of the bottom tape while the bottom tape lost the charge in the process. Through this, I also concluded that if they were both pieces of tape, they would have the same number/kind of particles, so there charges would remain the same if they didn't already create a charge. Hence, I think it is possible that the kind of particles a substance has can determine its charge.
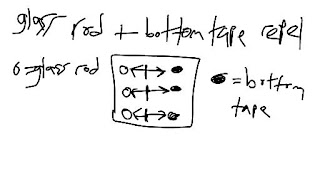
Charges occur when the electrons transfer from one place to another. Depending on the charges, they could charge with other things. This is what I learned in the sticky tape lab. In this lab, we tested two pieces of tape: one tape taped onto another tape. The objective was to test the top tape and the bottom tape with other things to determine whether they would repel or attract. The top tape that was ripped off the bottom tape seemed to attract to the bottom tape. But, why? I at first thought that the top tape got charge after tearing it off of the bottom tape while the bottom tape lost the charge in the process. Through this, I also concluded that if they were both pieces of tape, they would have the same number/kind of particles, so there charges would remain the same if they didn't already create a charge. Hence, I think it is possible that the kind of particles a substance has can determine its charge.Next, the bottom tape was tested with the paper and the aluminum. The bottom tape attracted to the aluminum, but repelled the glass rod. Hence, the aluminum must be positive while the glass rod was negative. Through this example, I learned that opposite charges attract while the same charges repelled–much like magnets. This is so because if the bottom tape and the top tape attracted together, they have opposite charges because when the top tape was ripped from the bottom tape, the top tape became positive while the bottom tape became negative. So, if the top tape is positive, then it would attract to something negative, like the glass rod, for example.
I then thought to myself whether charges and the flow of electrons have to do with electricity. I thought that this was the case because in the electrolysis lab, I learned that the cathobe and the anobe connected to the metal ends at the bottom of the trough, hence allowing electricity to flow through in order for the H2 and the O2 to separate from water. As I thought about this, I thought that metal was a conductor of electricity, and I also realized that oxygen and hydrogen had different charges. Otherwise, hydrogen and oxygen wouldn't have been separated from water. I then remember that the trough was made of glass, and knowing that it has a negative charge, I concluded that it couldn't allow electricity to flow through, whereas, metal could conduct electricity through the exchange of electrons.
This was the generalization I came to. To prove this, my group and I tested the conductivity (the ability to transfer electrons) of certain materials, such as: brass, silver, iron, zinc, copper, plastic, cardboard, and glass. The equipment we used included two battery sets, wire clips, and a light bulb. With the generalization I came up with, I predicted that the metal products would be conductive while the nonmetals would not be conductive. This prediction was correct because when all the metal products were connected to the wires, the batteries, and the light bulb, the light bulb lit, hence the electrons flowed through the wires to the light bulb. The nonmetal products, however, did not conduct electricity because the light bulb did not light up.



No comments:
Post a Comment